email: calmansys@yahoo.com
Temperature Measurement
Importance of Temperature Measurement
Temperature is one of the most frequently used process measurements.
Almost all chemical processes and reactions are temperature-dependent.
Frequently in chemical plants, temperature is the only indication of the progress of the process.
Early Measuring Devices
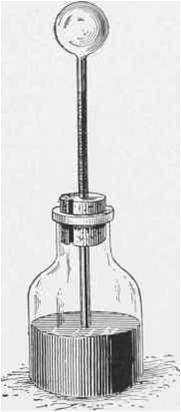
Galileo is credited with inventing the thermometer, 1592. In an open container
filled with colored alcohol, he suspended a long narrow-throated glass tube,
at the upper end of which was a hollow sphere.
When heated, the air in the sphere expanded and bubbled through the liquid.
Cooling the sphere caused the liquid to move up the tube.
In 1700 Gabriel Fahrenheit, a Dutch instrument maker, produced accurate and repeatable mercury thermometers.
For the fixed point on the low end of his temperature scale,
Fahrenheit used a mixture of ice water and salt(or ammonium chloride).
This was the lowest temperature he could reproduce, and he labeled it “zero degrees.”
For the high end of his scale, he chose human blood temperature and called it 96 degrees.
Why 96 and not 100 degrees? Earlier scales had been divided into twelve parts.
Fahrenheit, in an apparent quest for more resolution divided his scale into 24,
then 48 and eventually 96 parts.
The Fahrenheit scale gained popularity primarily because of the repeatability and
quality of the thermometers that Fahrenheit built.
Around 1742, Anders Celsius proposed that the melting point of ice and the boiling
point of water be used for the two benchmarks.
Celsius selected zero degrees as the boiling point and 100 degrees as the melting point.
Later, the end points were reversed and the centigrade scale was born.
In 1948 the name was officially changed to the Celsius scale.
In the early 1800’s William Thomson (Lord Kelvin), established the concept of absolute zero, and his scale remains the standard for modern thermometry.
What is Temperature?
Temperature is defined as the measure of heat (thermal energy) associated with the movement
(kinetic energy) of the molecules of a substance. This definition is based on the theory
that molecules of all matter are in continuous motion that is sensed as heat.
Heat in matter is due to movement at an atomic or molecular scale.
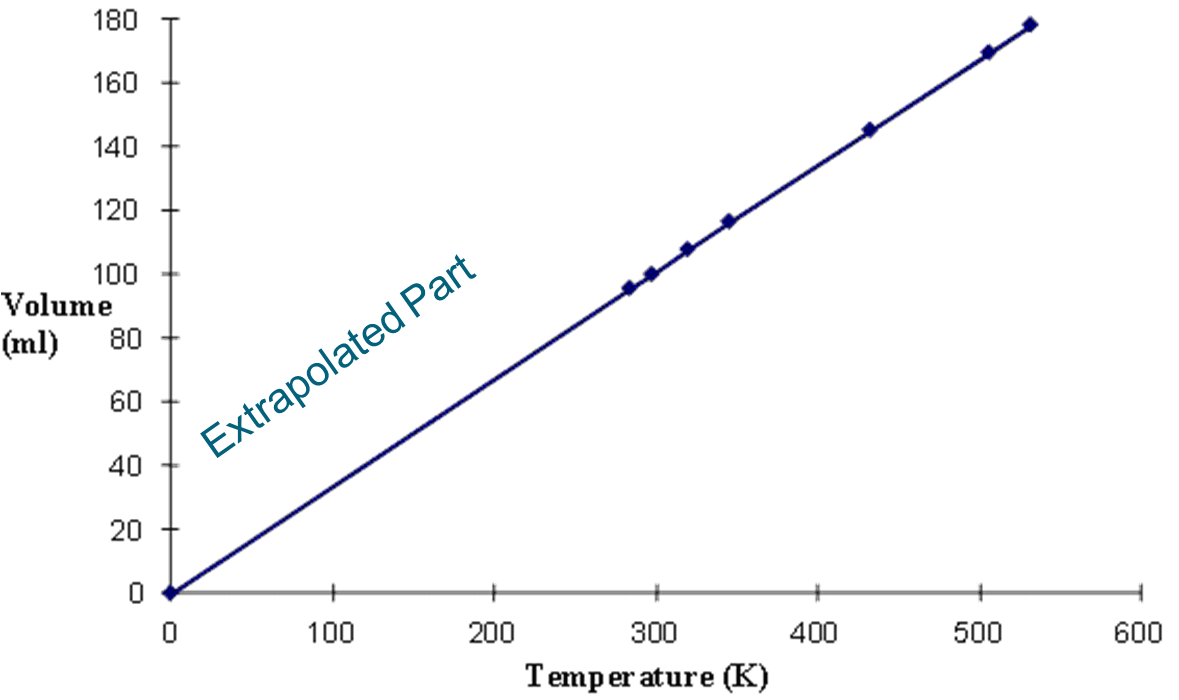
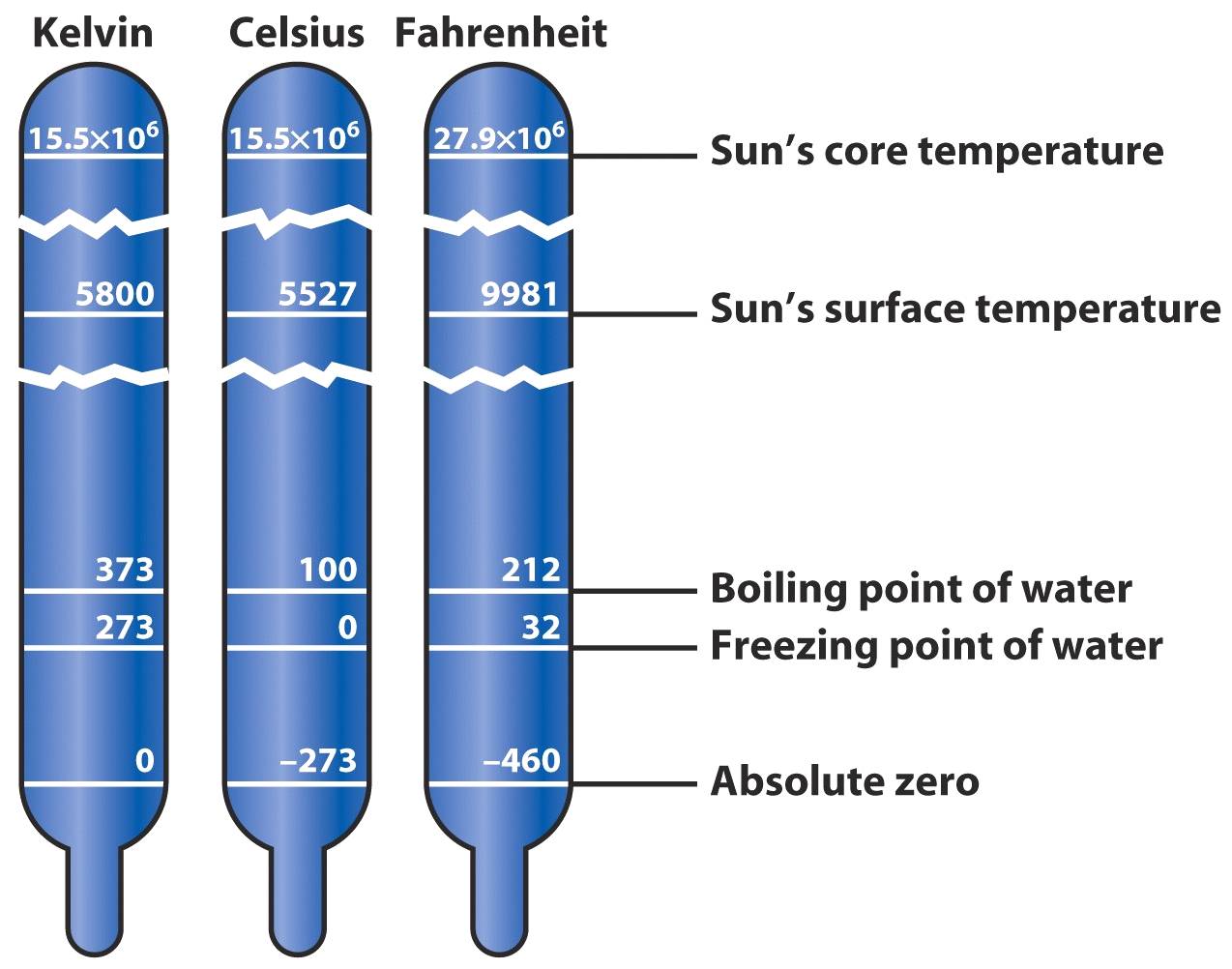
The temperature where all motion stops is called absolute zero Temperature.
This point is reached at -273°C.
This has never been achieved experimentally but was determined through
extrapolation of the linear temperature vs. volume relationship of an ideal gas.
Temperature Scales Conversion
Deg. F = 1.8 oC + 32
Deg. C = oF - 32 / 1.8
We cannot build a temperature divider as we can a voltage divider,
nor can we add temperatures as we would add lengths to measure distance.
We must rely upon temperatures established by physical phenomena
which are easily observed and consistent in nature.
The International Practical Temperature Scale (ITS 90) is based on such phenomena.
ITS 90
| Element |
|
Type |
K |
°C |
| (H2) |
Hydrogen |
Triple Point |
13.8033 K |
-259.3467° C |
| (Ne) |
Neon |
Triple Point |
24.5561 K |
-248.5939 ° C |
| (02) |
Oxygen |
Triple Point |
54.3584 K |
-218.7916° C |
| (Ar) |
Argon |
Triple Point |
83.8058 K |
-189.3442° C |
| (Hg) |
Mercury |
Triple Point |
234.315 K |
-38.8344° C |
| (H2O) |
Water |
Triple Point |
273.16 K |
+0.01° C |
| (Ga) |
Gallium |
Melting Point |
302.9146 K |
29.7646° C |
| (In) |
Indium |
Freezing Point |
429.7485 K |
156.5985° C |
| (Sn) |
Tin |
Freezing Point |
505.078 K |
231.928° C |
| (Zn) |
Zinc |
Freezing Point |
692.677 K |
419.527° C |
| (Al) |
Aluminum |
Freezing Point |
933.473 K |
660.323° C |
| (Ag) |
Silver |
Freezing Point |
1234.93 K |
961.78° C |
| (Au) |
Gold |
Freezing Point |
1337.33 K |
1064.18° |
Heat and Temperature
Heat and Temperature are often used interchangeably, but their
meanings are not the same. Heat is the thermal energy of a substance.
Addition or removal of it causes temperature to increase or decrease.
Unit of Heat
Heat is measured in units other than degrees. Two units often used to measure heat.
British Thermal Unit (BTU) and the Calorie.
A BTU is the quantity of heat required to raise the temperature of 1 pound of water one degree Fahrenheit.
A Calorie is the quantity of heat required to raise the
temperature of one gram of water one Degree Celsius.
Heat Transfer
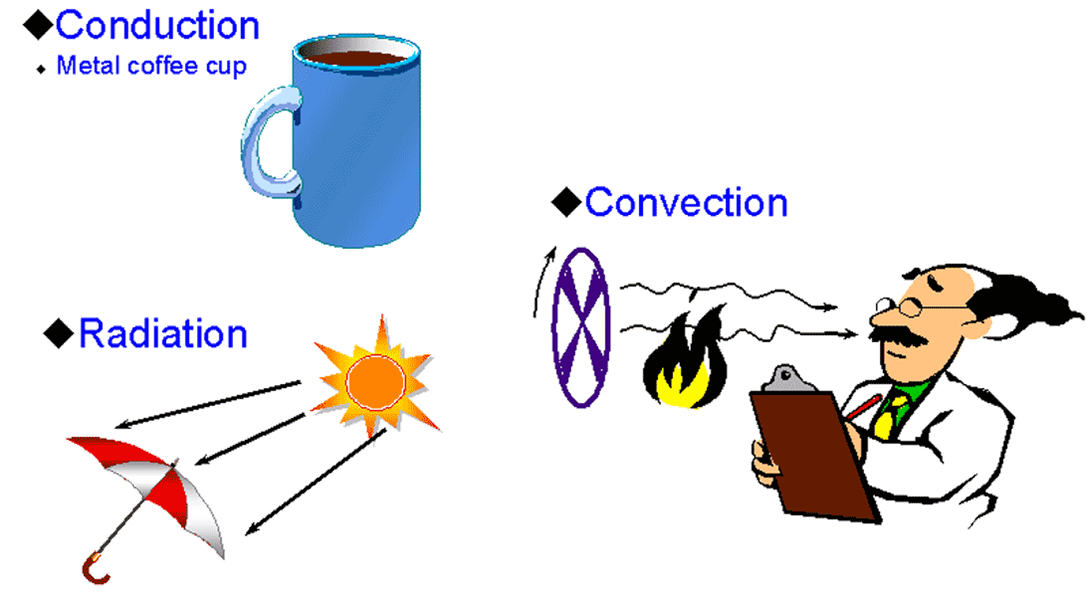
Heat is transferred between and within substances in one of the following ways:
Conduction, Convection and Radiation.
Conduction - is heat transfer through or between solids by the direct contact of molecules in those solids.
Thermal energy is transferred from one molecule to another when two molecules bump each other.
Ex: One end of metal bar is heated, the heat passes from one molecule to the other until the opposite end is also heated
Convection - Heat transfer involves the molecular movement of fluid masses
(liquid or gas) instead of the movement of molecules within solids.
Ex: Forced air heating system in which air is heated by furnace and travels through
the building in ducts warming the air in the rooms which it travels.
Radiation - Heat is transferred in the form of radiant energy through empty space.
Ex: Sun heats the earth by radiation.
Second Law of Thermodynamics
The second law of thermodynamics states that heat always flows
from a material at a high temperature to a material at lower temperature.
If the two bodies are in thermal equilibrium and no thermal energy
is exchanged, the bodies are at the same temperature.
Temperature Measuring Instruments
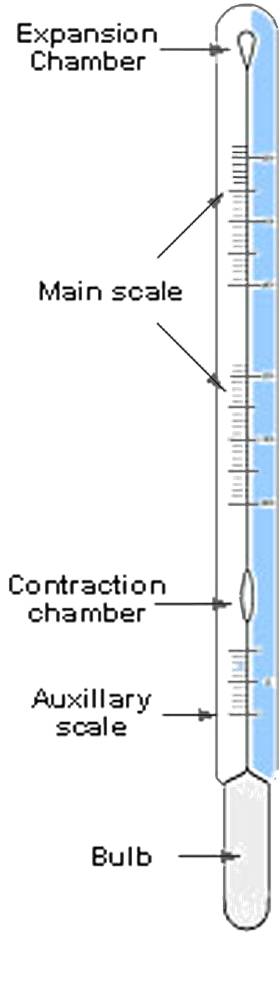
Fluid Thermometers: (Liquid in Glass)
Most commonly used type of mechanical thermometer is the fluid,
or liquid-in-glass thermometer. They are both used at home or
in an industrial facility.
Liquid-in-Glass Thermometer
Fluids Used in Fluid Thermometer:
1. Mercury : -39 to 600 oC
2. Mercury Alloys : -60 to 120
3. Alcohol : -80 to 100
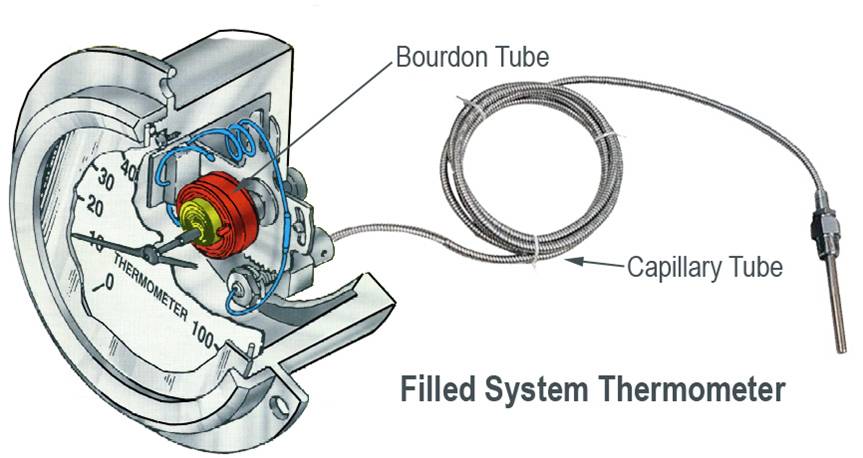
Filled System Thermometers
Filled Systems Thermometer - A temperature device that is filled a liquid,
gas, or vapor that will increase or decrease pressure on Bourdon tube,
bellows, or diaphragm in response to changes in temperature.
Filled System Thermometer
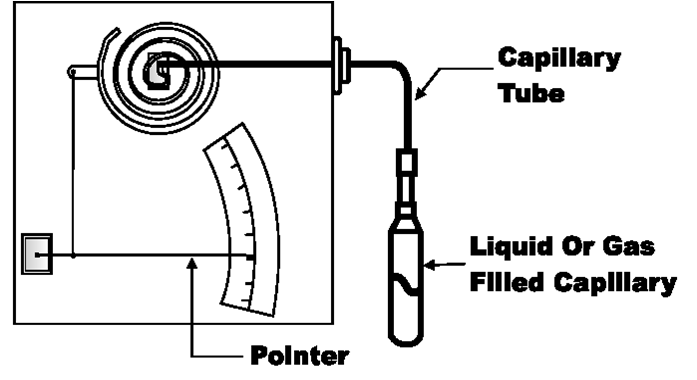
Comparison of Filled Systems
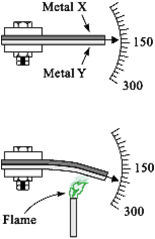
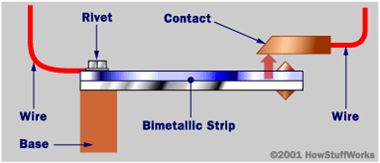
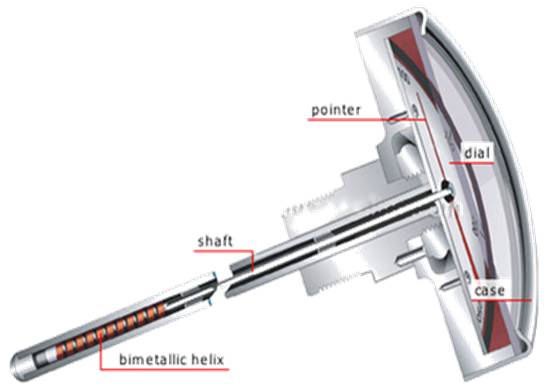
Bi-metallic Thermometers
Bi-metallic Thermometers are temperature measuring device that
contains a solid bimetallic element that bends in a predetermined
direction in response to changes in temperature.
Bimetallic Thermometer
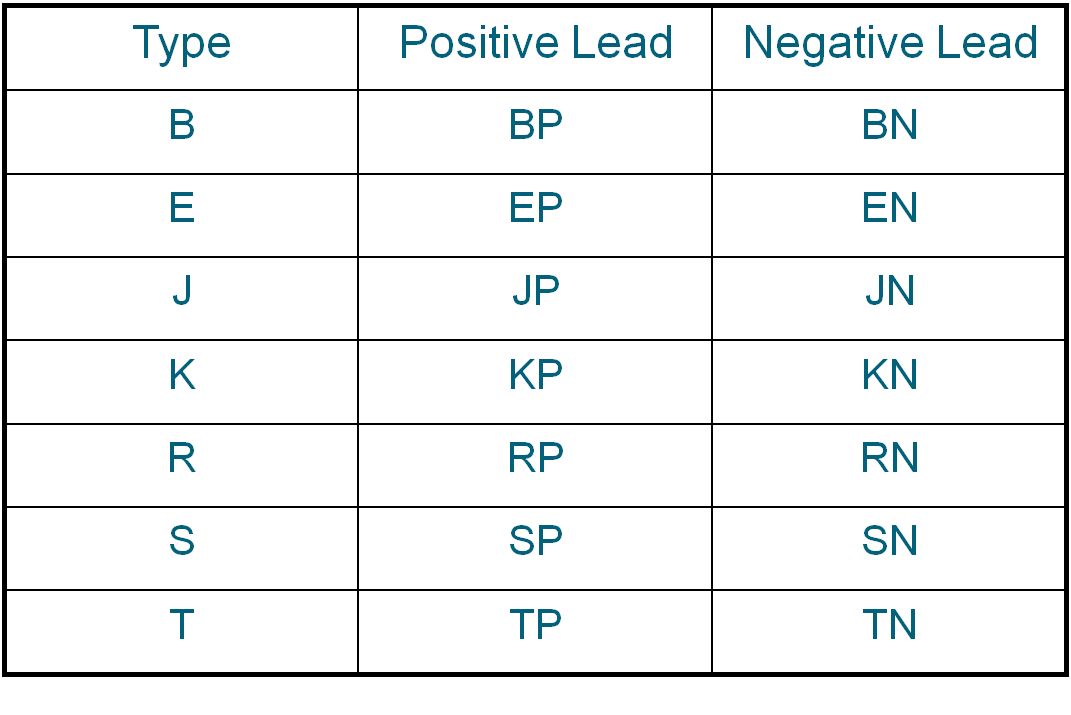
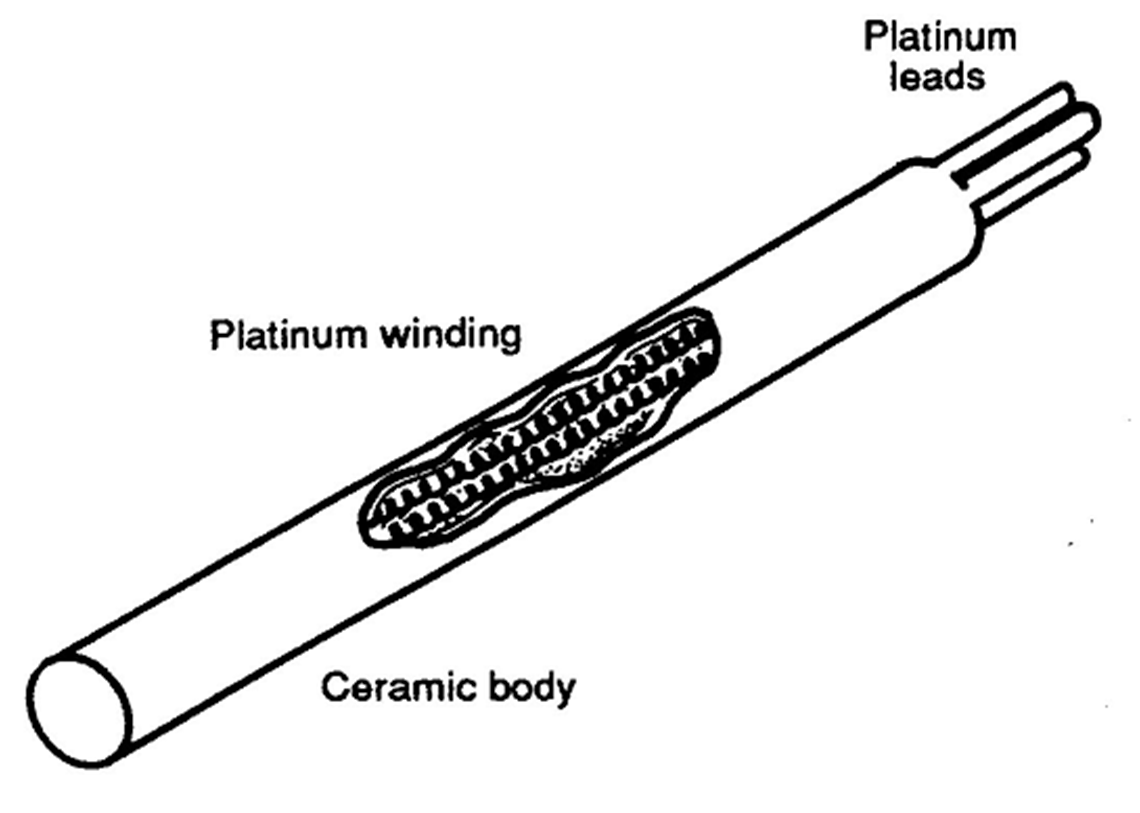
Thermocouple
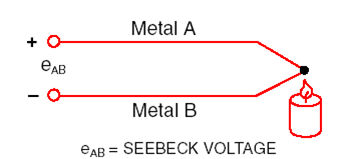
Thermocouple:
When two wires composed of dissimilar metals are joined at both ends and
one of the ends is heated, there is a continuous current which flows in the
thermoelectric circuit.
Thomas Seebeck made this discovery in 1821
Measuring Thermocouple Voltage
We can’t measure the Seebeck voltage directly because we must
first connect a voltmeter to the thermocouple, and thevoltmeter
leads themselves create a new thermoelectric circuit.
Let’s connect a voltmeter across a copper- constantan (Type T)
thermocouple and look at the voltage output:
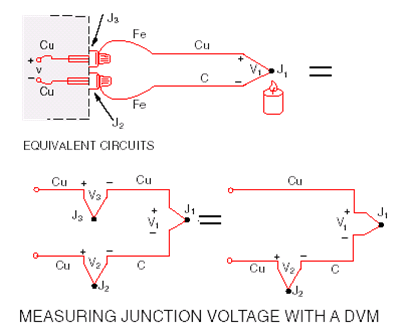
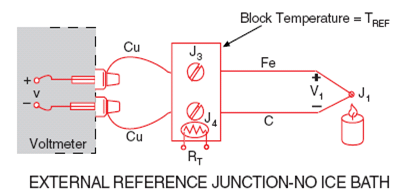
We would like to read only V1, but by connecting the voltmeter
to measure the output of Junction J1, we have created two
more metallic junctions: J2 and J3. J3 is a copper-to-copper junction,
it creates no thermal EMF (V3 = 0), but J2 is a copper-to-constantan
junction which will add an EMF (V2) in opposition to V1.
The voltmeter reading V will be proportional to the temperature difference between J1 and J2. We can’t determine the temperature at J1 unless we first find the temperature of J2.
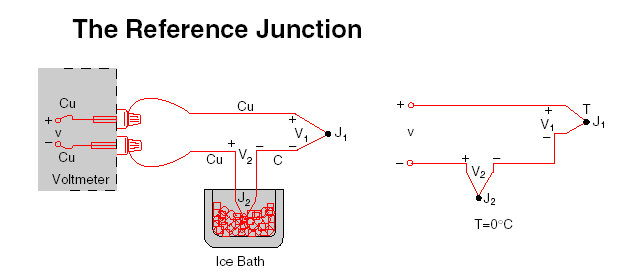
One way to determine the temperature of J2 is to physically put the
junction into an ice bath, forcing its temperature to be 0°C and establishing J2 as the Reference Junction. Since both voltmeter terminal junctions are now copper-copper, they create no thermal emf and the reading V on the voltmeter is proportional to the temperature difference between J1and J2.
Steps in Cold Junction Automatic Cold Junction Compensation
1. Measure Cold Junction Temperature and note the voltage from
T/C table corresponding to that temperature.
This is the Cold Junction Compensation voltage.
2. Measure V.
3. Add the Cold Junction Compensation voltage to V.
4. Convert V from T/C table.
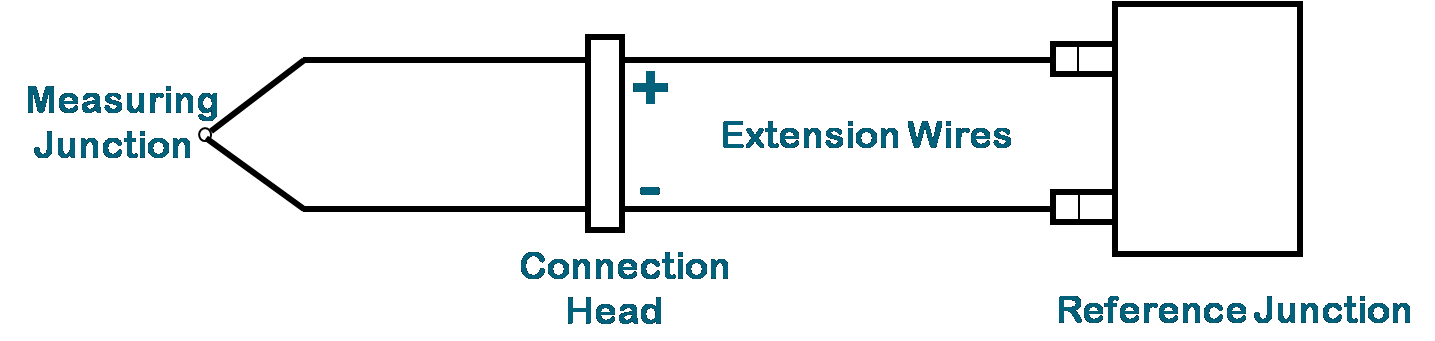
A separate junction is created whenever we connect a thermocouple
using ordinary wire.To avoid this, special extension wires designed
for the particular thermocouple should be used. The extension wire
in effect brings the thermocouple junction to the indicator terminals
where cold junction compensation is realized.
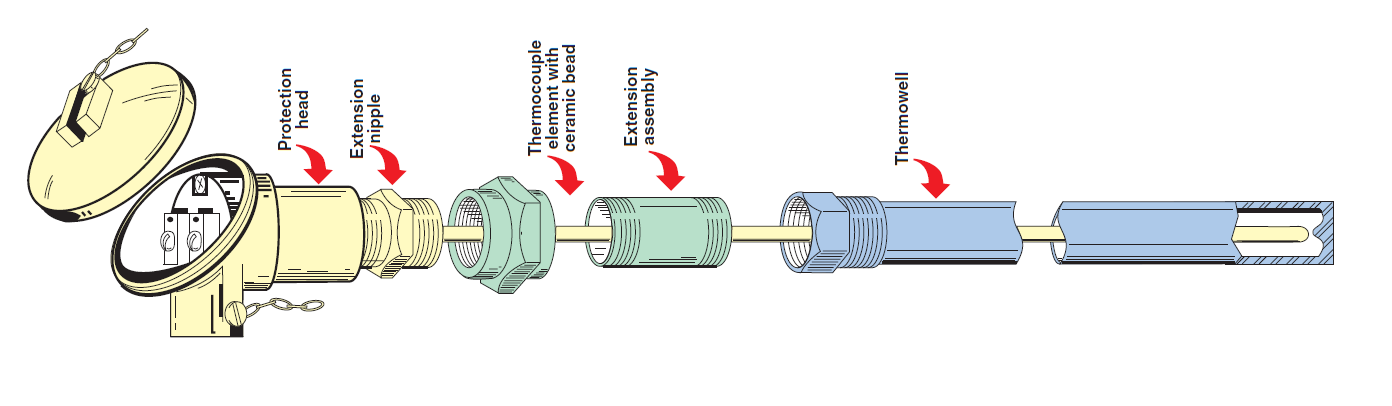
Thermocouple Circuit With Extension Wires
Thermocouple Circuit Assembly
Thermowells
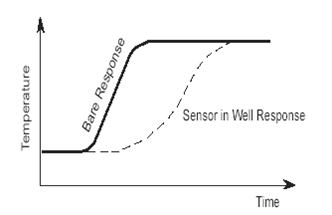
Thermocouple Well ResponseFunctional Classes of Thermocouples:
1. Base Metal Thermocouples
2. Noble Metal Thermocouples
3. Refractory Metal Thermocouples
Base Metal Thermocouples:
Useful for measuring temperatures under 1000 Deg. C.
This class includes:
• Iron/Constantan (Type J)
• Copper/Constantan (Type T)
• Chromel/Alumel (Type K)
• Chromel/Constantan (Type E)
• Alloys of copper, nickel, iron, chromium, manganese and other elements.
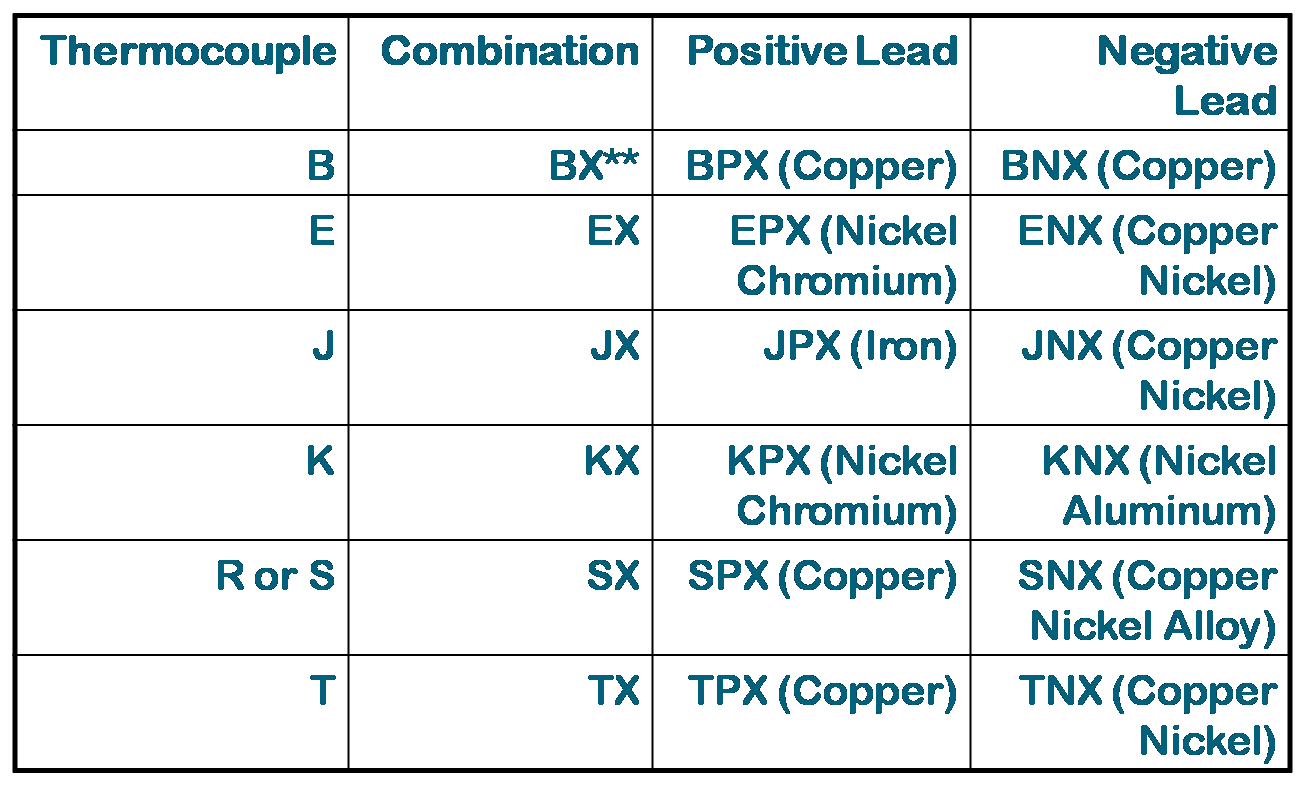
Symbols for Types of Extension Wires
Both types R or S Thermocouples use the same SX compensating extension wires.
Duplex Insulated Thermocouple Wires
* A tracer color of the positive wire code color maybe used in the overall braid.
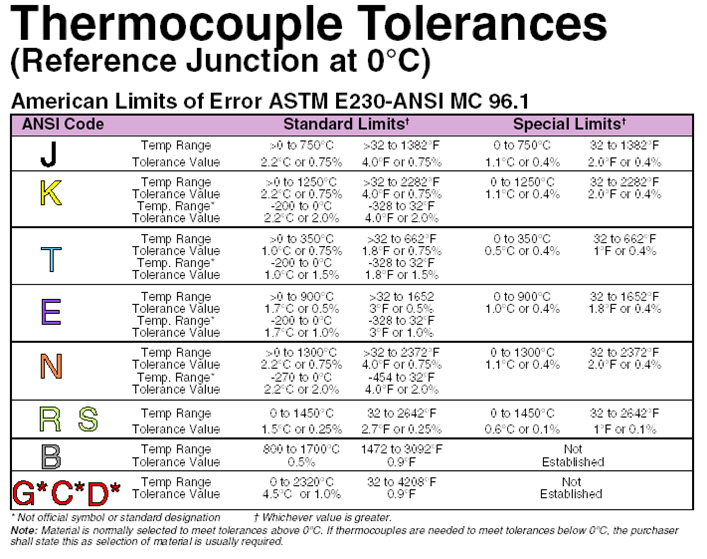
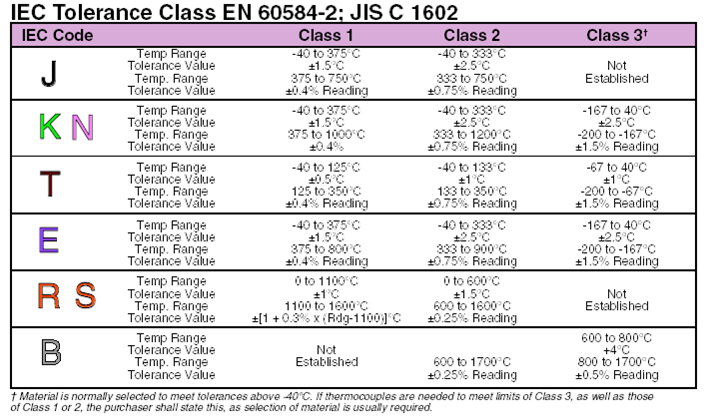
TABLE 9 Type K Thermocouple ? thermoelectric voltage as a function of temperature (°C);
reference junctions at 0 °C
°C 0 1 2 3 4 5 6 7 8 9 10 °C Thermoelectric Voltage in Millivolts
0 0.000 0.039 0.079 0.119 0.158 0.198 0.238 0.277 0.317 0.357 0.397
0
10 0.397 0.437 0.477 0.517 0.557 0.597 0.637 0.677 0.718 0.758 0.798
10
20 0.798 0.838 0.879 0.919 0.960 1.000 1.041 1.081 1.122 1.163 1.203
20
30 1.203 1.244 1.285 1.326 1.366 1.407 1.448 1.489 1.530 1.571 1.612
30
40 1.612 1.653 1.694 1.735 1.776 1.817 1.858 1.899 1.941 1.982 2.023
40
Thermowell Installation
Advantages of Thermocouple
Simple, rugged construction.
Has a wider temperature range.
Self-powered.
Wide variety.
Low cost.
Disadvantages of Thermocouple
Expensive extension grade thermocouple wires or compensating
cables have to be used.
Sensitive to induction interference.
Slower response.
Non- linear, least stable and least sensitive.
Reference required.
Failure Modes ofThermocouple
An open circuit in the thermocouple detector means there is no path
for current flow, thus it will cause a low temperature reading.
A short circuit in the thermocouple detector will cause a
low temperature reading because it creates a leakage current
path to the ground and a smaller measured voltage.
Thermocouple Signal Converter
Current Output Terminals

TC TerminalsThermocouple (E/I) Converter
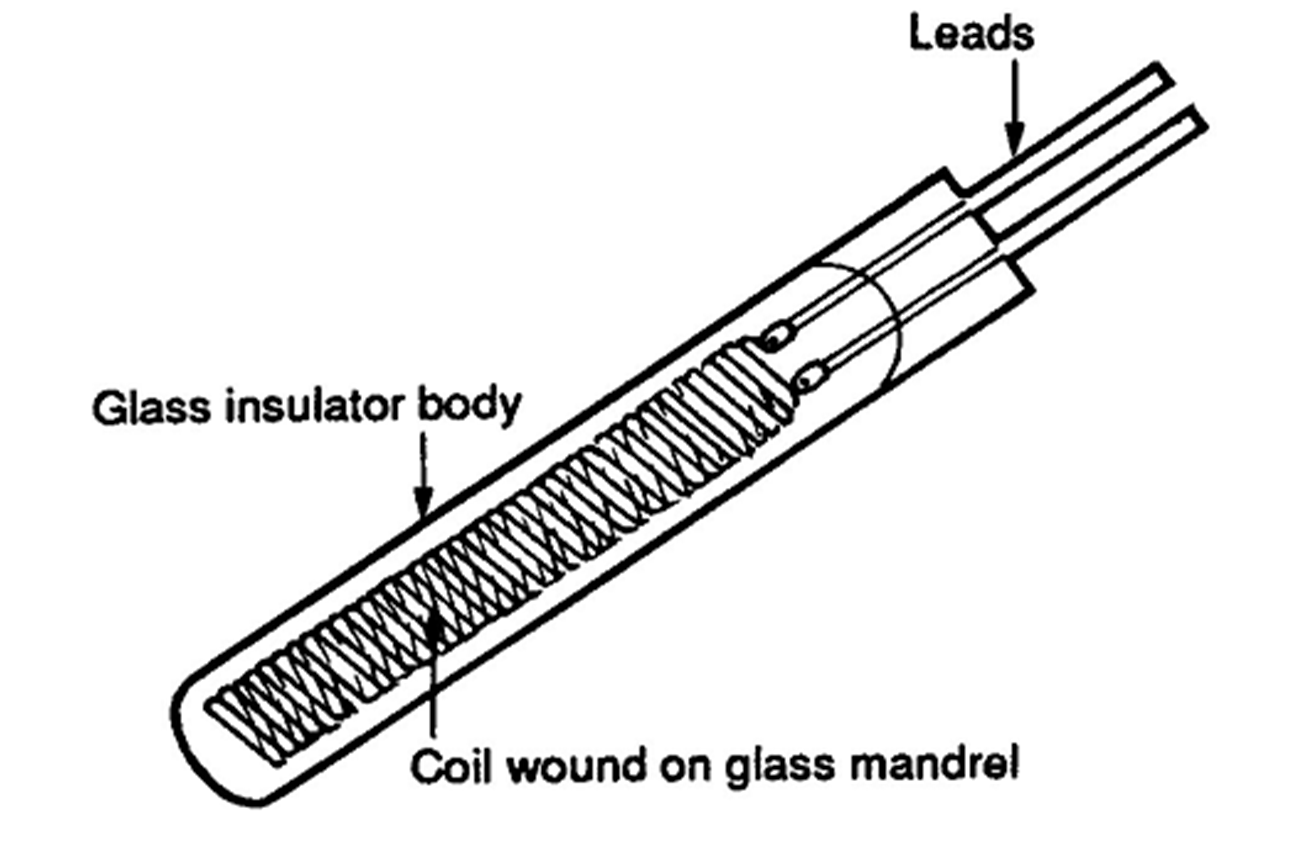
RTD (Resistance Temperature Detector)
An RTD is an electrical temperature sensor that uses changes
in resistance as the basis for temperature measurement.
The operation of an RTD is based on the fact that the electrical
resistance of some metals varies directly with temperature changes.
An RTD consist of a sensing element fabricated of metal wire which
responds to temperature change by changing its resistance
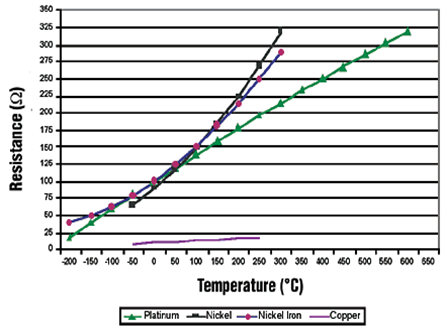
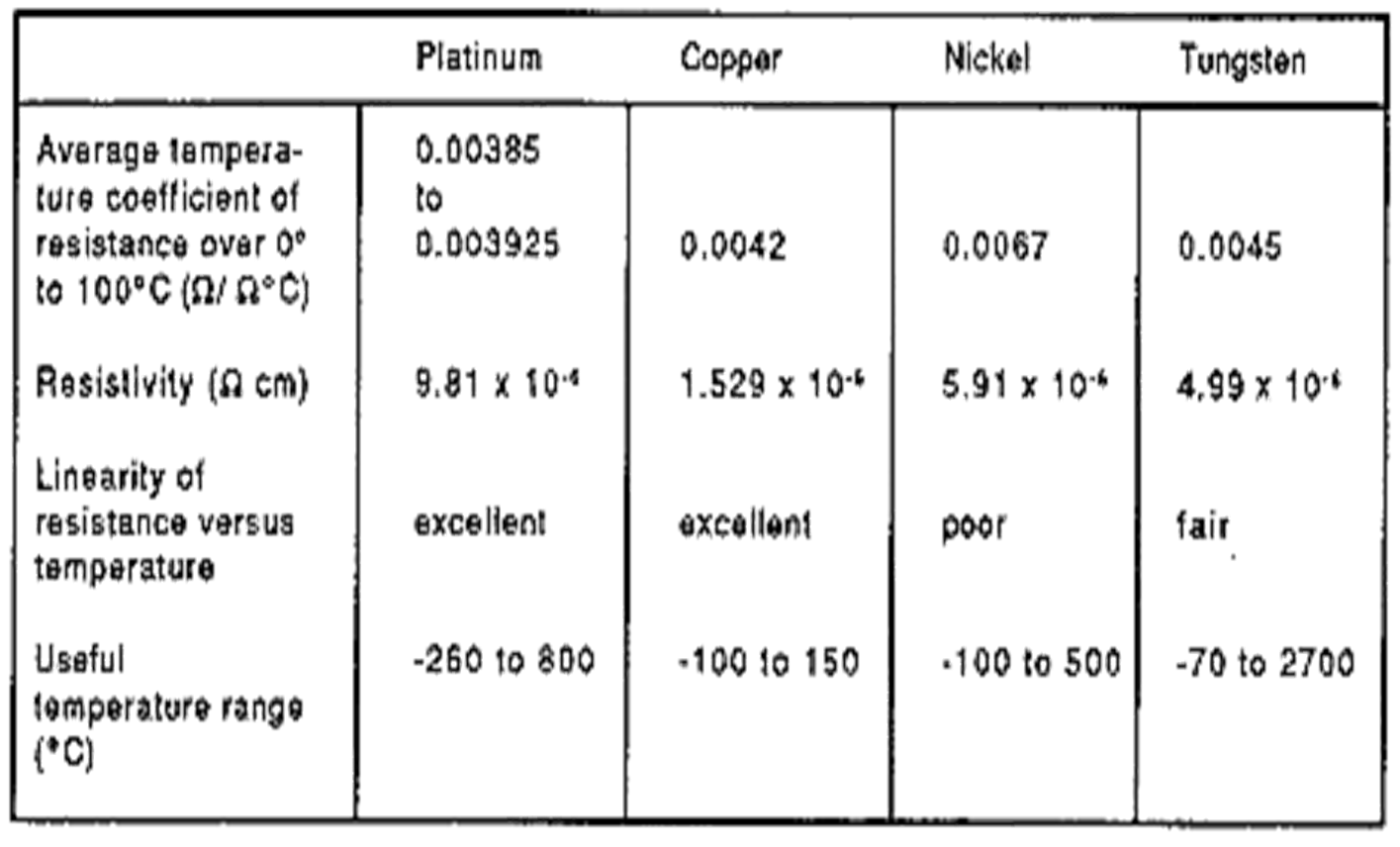
Resistance Vs. Temperature RTD Materials
Properties of Metals Used RTD's
Partially Supported RTD Leads
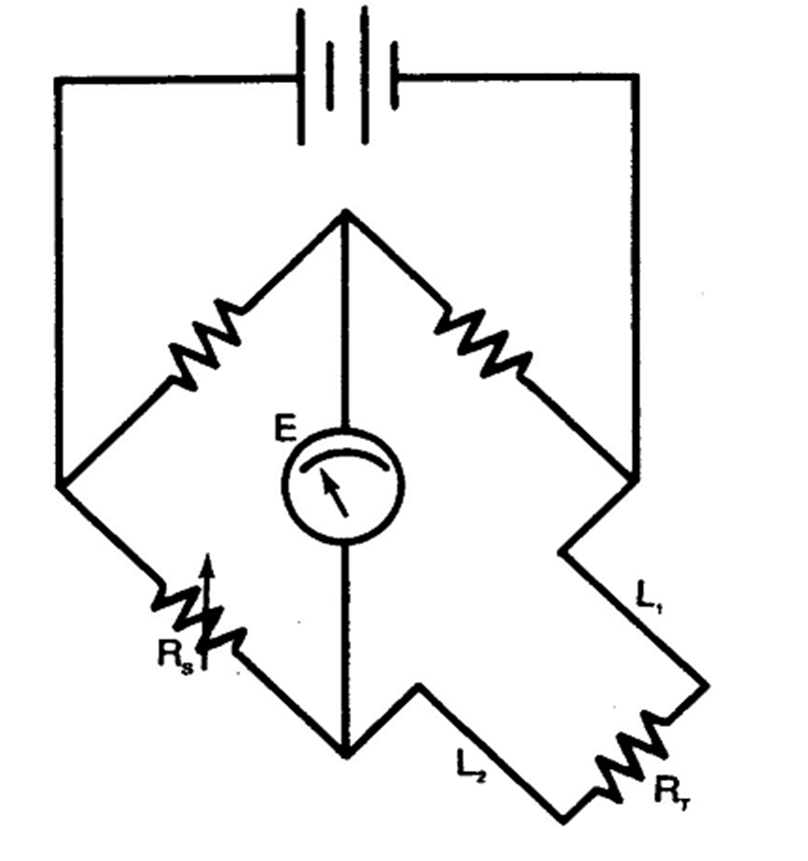
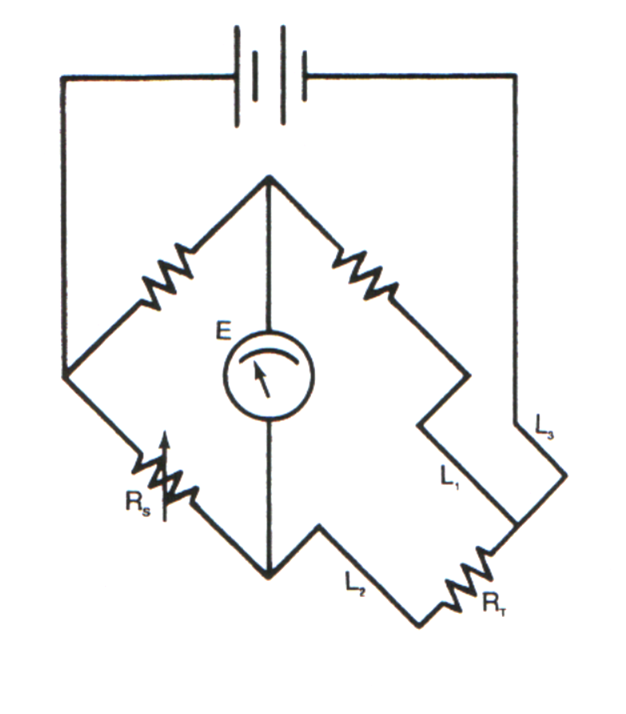
Two-wire RTD and Wheatstone Circuit
Two-wire RTD and Wheatstone Circuit
Two wire RTDs should not be used where the sensor is far from
the indicator because the connecting wires L1 and L2 adds to
the RTD resistance.
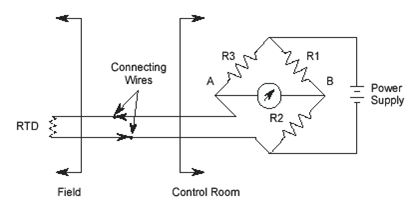
Three-wire RTD and Wheatstone Circuit
On a 3 wire RTD L2 is in series with the RTD
while the L1 is in series with th reference resistor.
The resistance of L1 & L2 cancels each other out.
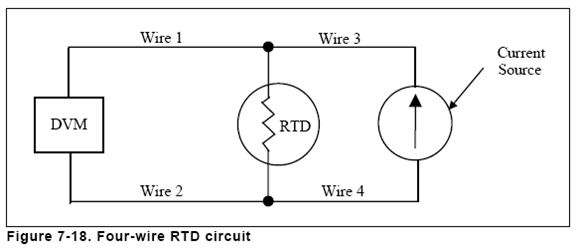
For highest accuracy, 4 wire configuration is used.
Figure 7-18. Four-wire RTD circuit
Advantages of RTD
The response time is faster. No affected by drift (Stable).
More accurate and has higher sensitivity.
More linear than thermocouple.
Does not require special extension cable.
Disadvantages of RTD
More expensive since the metal used is in purest form.
Limited measuring range.
A power supply failure can cause erroneous readings.
Sensitive to loose connections.
Self-heating.
Low absolute resistance.
Failure Modes of RTD
An open circuit in the RTD or in the wiring between the RTD
and the bridge will cause a high temperature reading.
Loss of power or a short within the RTD will cause a
low temperature reading.
RTD Signal Converter
(R/I Converter) Current Output Terminals
RTD Terminals
Thermistors
Thermistor is an electrical temperature measuring device made of semi-conductor
material whose resistance is inversely proportional to temperature.
Thermistors are semiconductors formed from complex metal oxides.
They are available with positive temperature coefficients (PTC) and with negative
temperature coefficient (NTC).
NTC thermistors are used exclusively for temperature measurement.
Thermistors
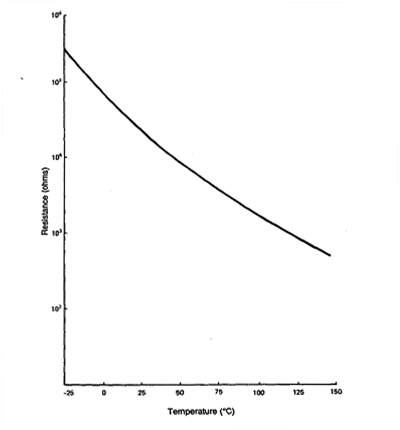
Resistance Vs. Temperature for Typical Thermistor
Common ThermistorDesign
Advantages of a Thermistor
The response time is faster than RTD & TC.
Two-wire resistance measurement.
Disadvantages of aThermistor
Non-linear response.
Limited temperature range.
Very fragile.
Current source required.
Self-heating.
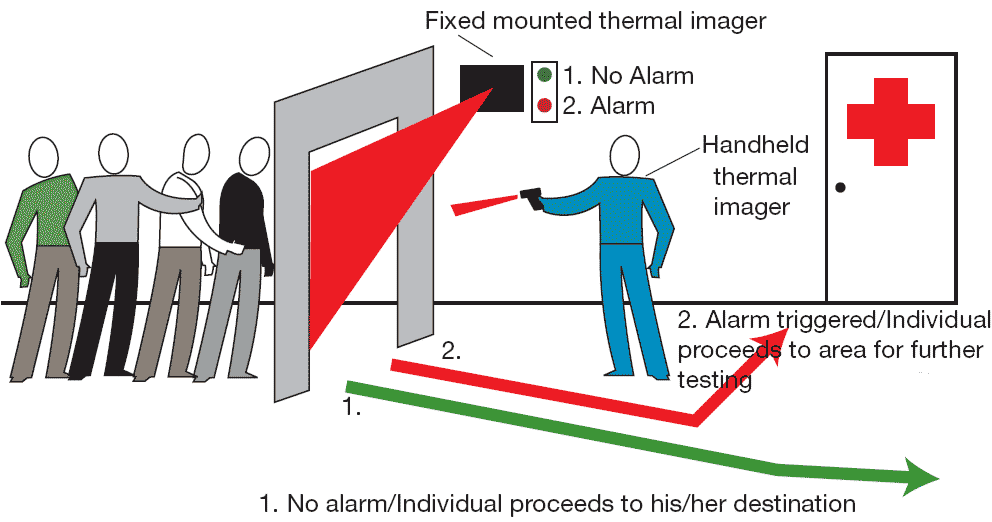
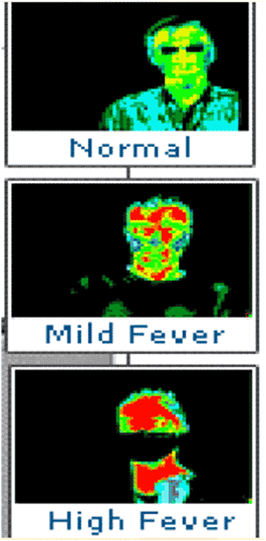
Infrared Thermometer
An infrared thermometer is a non-contact temperature measurement device.
Infrared Thermometers detect the infrared energy emitted by all materials
-- at temperatures above absolute zero, (0°Kelvin)-- and converts the
energy factor into a temperature reading.
How do infrared thermometers work?
The basic design consists of a lens to focus the infrared (IR) energy on
to a detector, which converts the energy to an electrical signal that can
be displayed in units of temperature after being compensated for ambient
temperature variation. This facilitates temperature measurement without
contact with the object.
Typical applications are where the object is moving; surrounded by an EM
field, as in induction heating; contained in a vacuum or other controlled
atmosphere; or where fast response is required.
Critical considerations for infrared pyrometer:
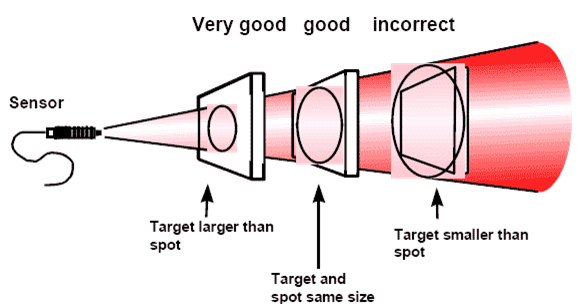
1. Field of view (target size and distance)
2. Type of surface being measured (emissivity considerations)
3. Spectral response (for atmospheric effects or transmission through surfaces)
4. Temperature range and mounting (handheld portable or fixed mount).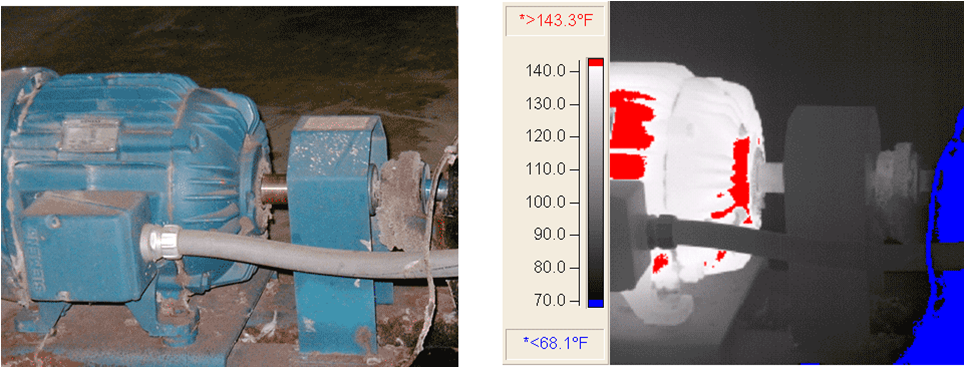
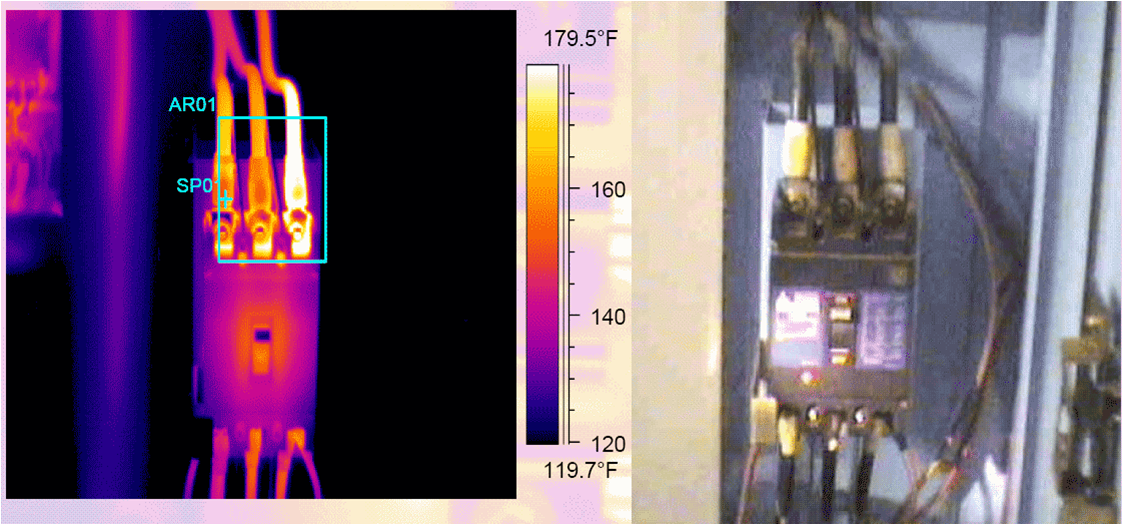
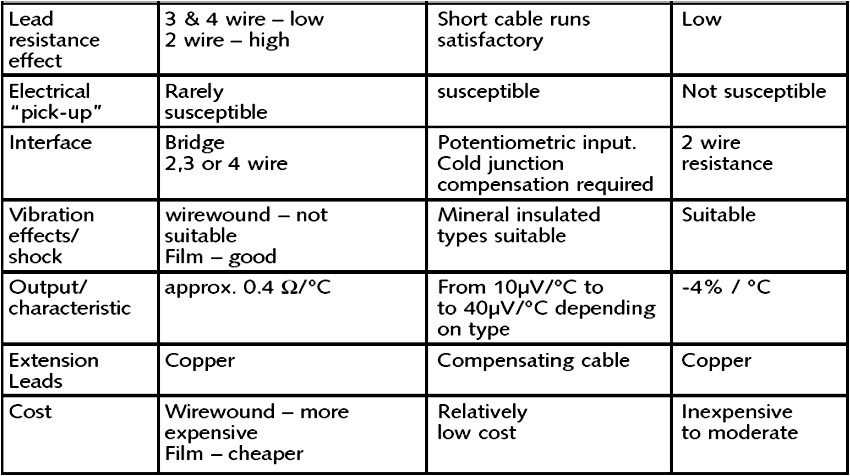
Thermal imaging used on equipmentComparison of Temperature Sensors
RTD Thermocouple Thermistor
email: calmansys@yahoo.com