
Benjamin S. Valle
email: calmansys@yahoo.com
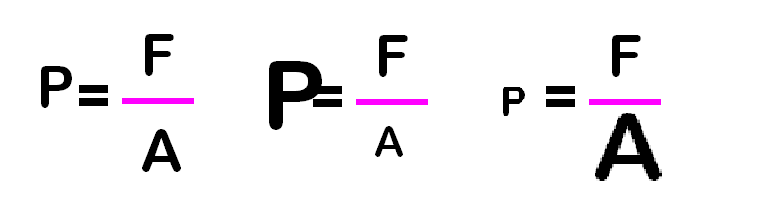
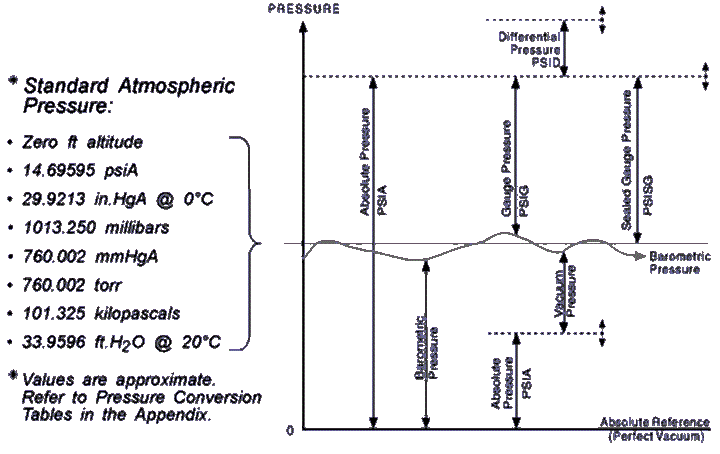
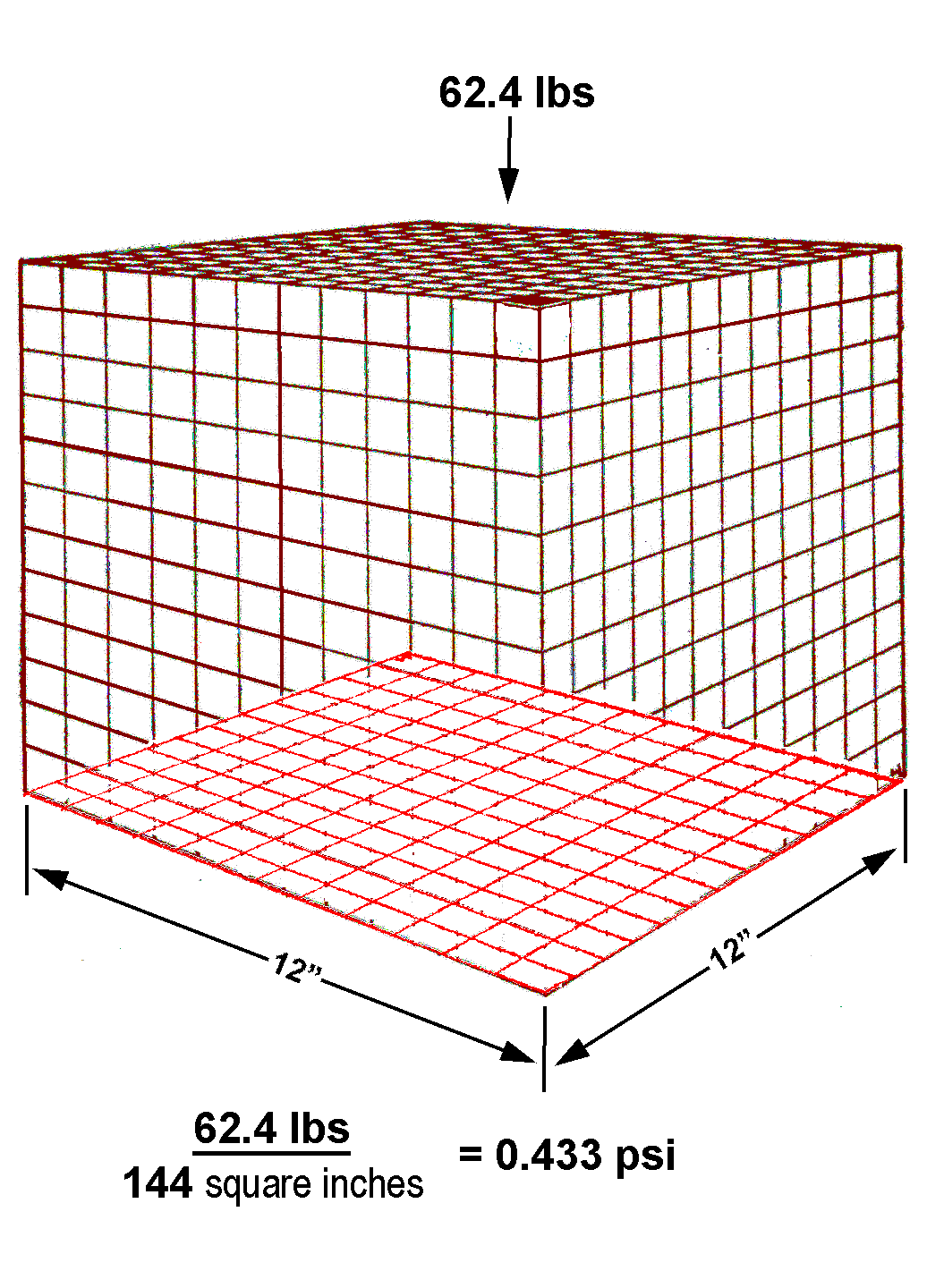
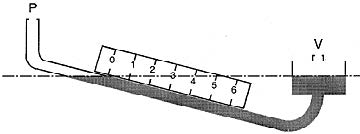
The question asks how many pounds per square inch does
the box exert. Put 62.4 pounds in the numerator and 144
square inches in the denominator, divide, and you get 0.433 psi
Multiply it by 20, we get the psi of the 20-foot-high column
Now we multiply that by 1.5 because the substance in this
problem has 1.5 times the mass of water (0.433 x 20 x 1.5 = 12.99)
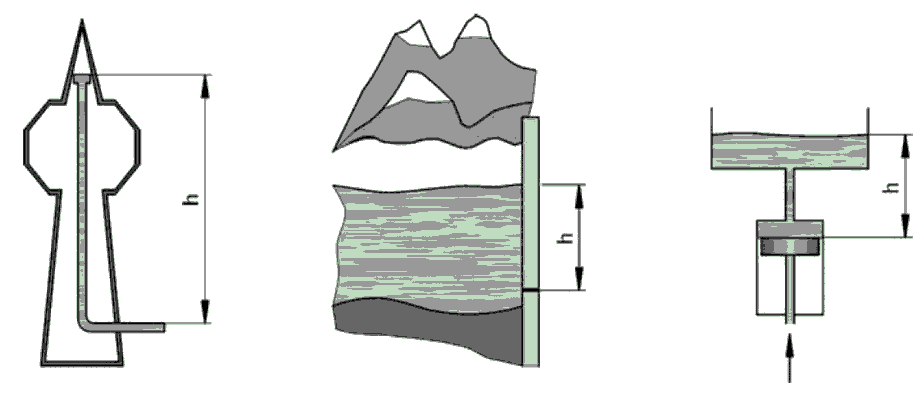
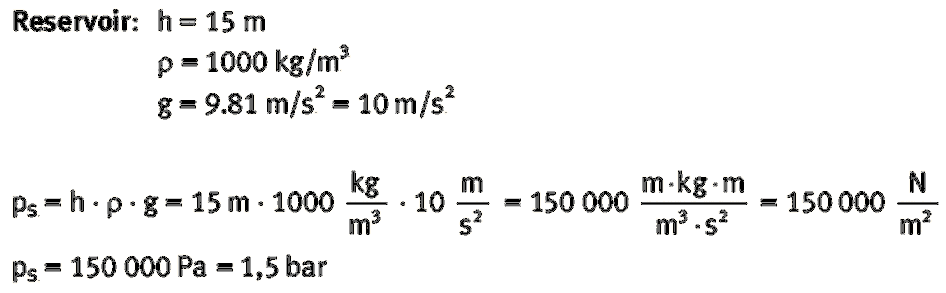
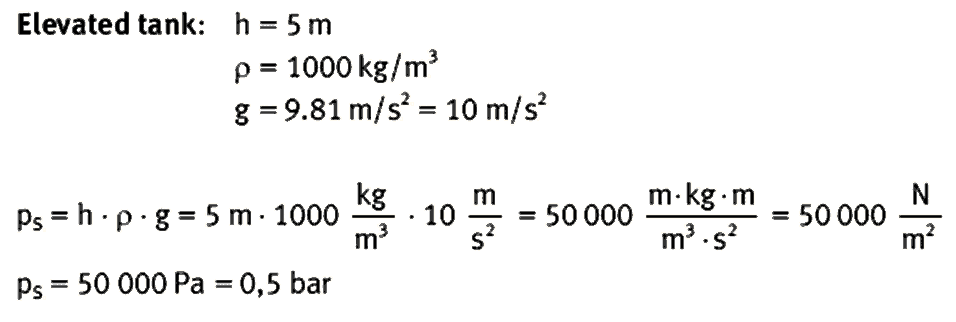
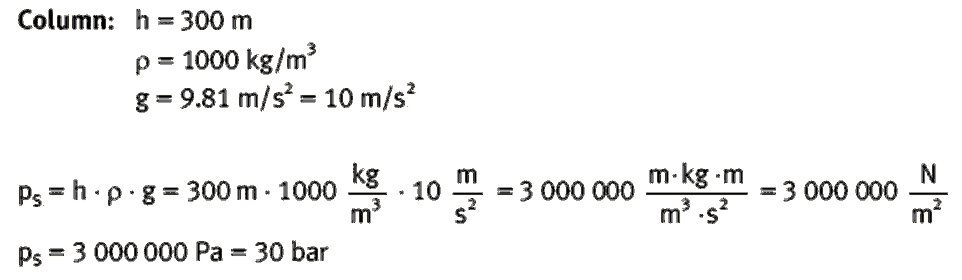
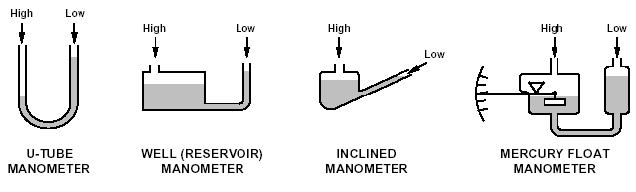

glass tube partially filled with liquid.
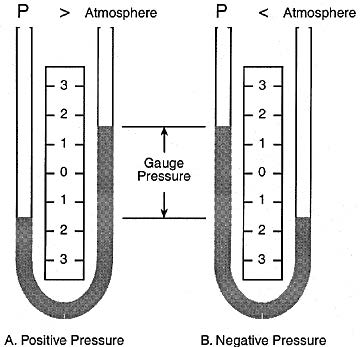
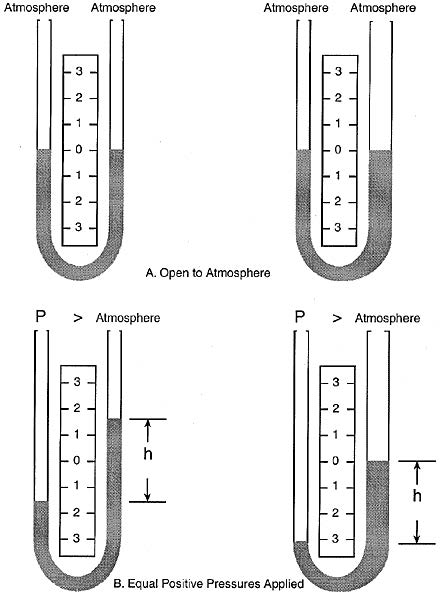
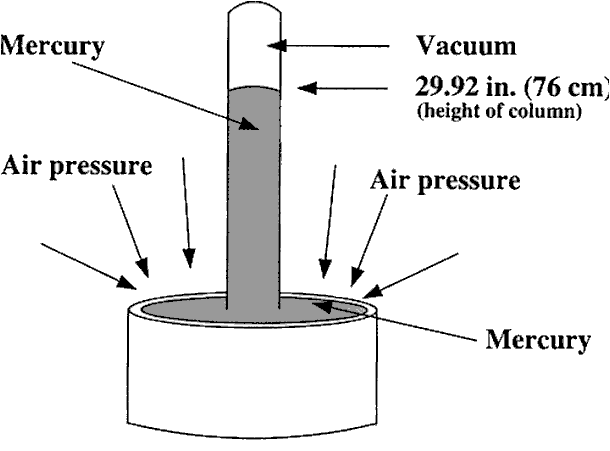
This manometer has no moving parts and requires no calibration.
Manometer measurements are functions of gravity and the liquid’s density, both physical properties that make the U-tube manometer a NIST standard for accuracy.
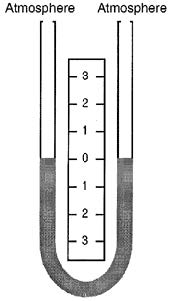
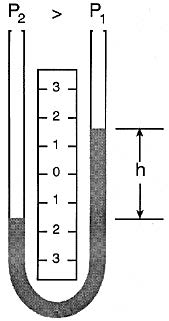
The liquid moves until the unit weight of the liquid, as indicated by h, exactly balances the pressure.
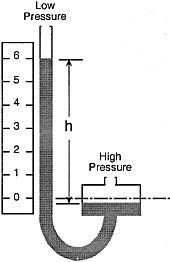
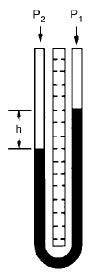 When the liquid in the tube is mercury, for example, the indicated pressure his usually expressed in inches of mercury. To convert to pounds per square inch, P2 = dh
When the liquid in the tube is mercury, for example, the indicated pressure his usually expressed in inches of mercury. To convert to pounds per square inch, P2 = dh